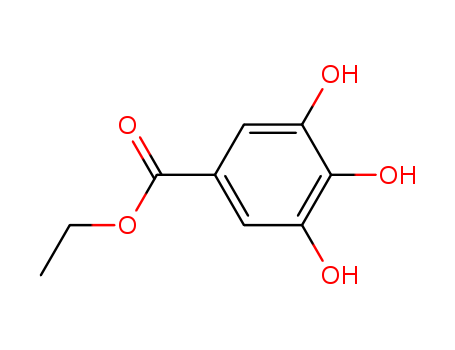
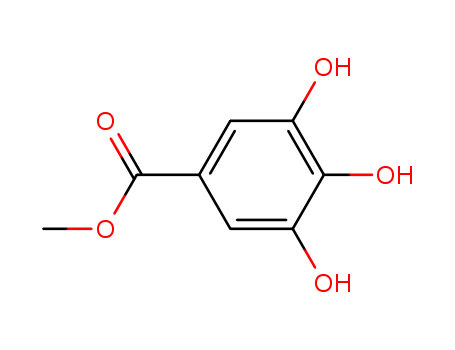
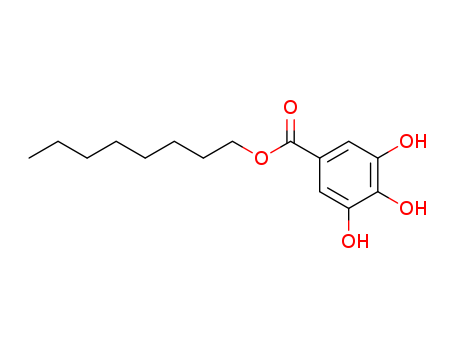
- Product Details
Keywords
- Chinese tannin
- Gallotannic acid
- 1,3,6-tris-O-(3,4,5-trihydroxybenzoyl)hexopyranose
Quick Details
- ProName: Tannic acid
- CasNo: 1401-55-4
- Molecular Formula: C76H52O46
- Appearance: Light yellow to light brown powder
- Application: Used as raw materials for making galli...
- PackAge: 25kg/woven bag
- ProductionCapacity: 1000 Metric Ton/Year
- Purity: 81.0%;86.0%;92.0%;96.0%;99.0%;
- Storage: Avoid moisture and light; hermetically...
- LimitNum: 0 Metric Ton
Superiority
Tannic acid is a specific form of tannin, a type of polyphenol. Its weak acidity (pKa around 6) is due to the numerous phenol groups in the structure. The chemical formula for commercial tannic acid is often given as C76H52O46, which corresponds with decagalloyl glucose, but in fact it is a mixture of polygalloyl glucoses or polygalloyl quinic acid esters with the number of galloyl moieties per molecule ranging from 2 up to 12 depending on the plant source used to extract the tannic acid. Commercial tannic acid is usually extracted from any of the following plant parts: Tara pods (Caesalpinia spinosa), gallnuts from Rhus semialata or Quercus infectoria or Sicilian Sumac leaves (Rhus coriaria).
According to the definitions provided in external references such as international pharmacopoeia, Food Chemicals Codex and FAO-WHO tannic acid monograph only tannins sourced from the above-mentioned plants can be considered as tannic acid. Sometimes extracts from chestnut or oak wood are also described as tannic acid but this is an incorrect use of the term. It is a yellow to light brown amorphous powder; 2850 gramsdissolves in one litre of water (1.7 moles per liter).
While tannic acid is a specific type of tannin (plant polyphenol), the two terms are sometimes (incorrectly) used interchangeably. The long-standing misuse of the terms, and its inclusion in scholarly articles has compounded the confusion. This is particularly widespread in relation to green tea and black tea, both of which contain tannin but not tannic acid.
Tannic acid is not an appropriate standard for any type of tannin analysis because of its poorly defined composition.
Details
annins are a basic ingredient in the chemical staining of wood, and are already present in woods like oak, walnut, and mahogany. Tannic acid can be applied to woods low in tannin so chemical stains that require tannin content will react. The presence of tannins in the bark of redwood (Sequoia) is a strong natural defense against wildfire, decomposition and infestation by certain insects such as termites. It is found in the seeds, bark, cones, and heartwood.
Tannic acid is a common mordant used in the dyeing process for cellulose fibers such as cotton, often combined with alum and/or iron. The tannin mordant should be done first as metal mordants combine well with the fiber-tannin complex. However this use has lost considerable interest.
Similarly tannic acid can also be used as an aftertreatment to improve wash fastness properties of acid dyed polyamide. It is also an alternative for fluorcarbon aftertreatments to impart anti-staining properties to polyamide yarn or carpets. However, due to economic considerations currently the only widespread use as textile auxiliary is the use as an agent to improve chlorine fastness, i.e. resistance against dye bleaching due to cleaning with hypochlorite solutions in high-end polyamide 6,6-based carpets and swimwear. It is, however, used in relatively small quantities for the activation of upholstery flock; this serves as an anti-static treatment.
Tannic acid is used in the conservation of ferrous (iron based) metal objects to passivate and inhibit corrosion. Tannic acid reacts with the corrosion products to form a more stable compound, thus preventing further corrosion from taking place. After treatment the tannic acid residue is generally left on the object so that if moisture reaches the surface the tannic acid will be rehydrated and prevent or slow any corrosion. Tannic acid treatment for conservation is very effective and widely used but it does have a significant visual effect on the object, turning the corrosion products black and any exposed metal dark blue. It should also be used with care on objects with copper alloy components as the tannic acid can have a slight etching effect on these metals.
Tannic acid is also found in commercially available iron/steel corrosion treatments, such as Hammerite Kurust.
Use in food
Use of tannic acid in food applications is far more widespread and significant amounts are used as process aids in beer clarification, aroma compound in soft drinks and juices. Equally important are applications in the wine industry, where it finds use as a natural clarifying agent, colour stabilizer and taste enhancer.
In many parts of the world, such uses are permitted. In the United States, tannic acid is generally recognized as safe by the Food and Drug Administration.
According to EU directive 89/107/EEC tannic acid cannot be considered as a food additive and consequently does not hold an E number. Under directive 89/107/EEC tannic acid can be referred to as a food ingredient. The E-number E181 is sometimes incorrectly used to refer to tannic acid; this in fact refers to the INS number assigned to tannic acid under the FAO-WHO Codex Alimentarius system.
Uses as a medication
In conjunction with magnesium and sometimes activated charcoal, tannic acid was once used as a treatment for many toxic substances, such as strychnine, mushroom, and ptomaine poisonings in the late 19th and early 20th centuries.
The introduction of tannic acid treatment of severe burn injuries in the 1920s significantly reduced mortality rates.During World War I, tannic acid dressings were prescribed to treat "burns, whether caused by incendiary bombs, mustard gas, or lewisite. After the war this use was abandoned due to the development of more modern treatment regimens.
Tannic acid is still used in pharmaceutical applications to produce albumin tannate which is used as an antidiarrheal agent. Tannic acid is also used to produce tannate salts of certain antihistamine and antitussive products to impart increased stability or slow release properties to the active pharmaceutical ingredient. Further to this, tannic acid is the principle but perhaps minimally effective ingredient in antiallergen sprays.
Tannins have also been reported to exert many physiological effects, such as to accelerate blood clotting, reduce blood pressure, decrease the serum lipid level, produce liver necrosis, and modulate immunoresponses. This would explain common folklore such as that soaking feet in tannic acid (or strong tea) can treat or prevent blisters, foot odor and rough, dry feet.


 Premiumsupplier
Premiumsupplier