- Product Details
Keywords
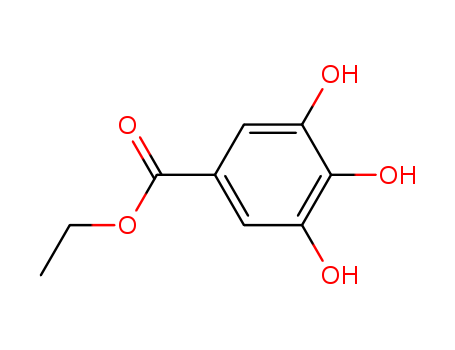
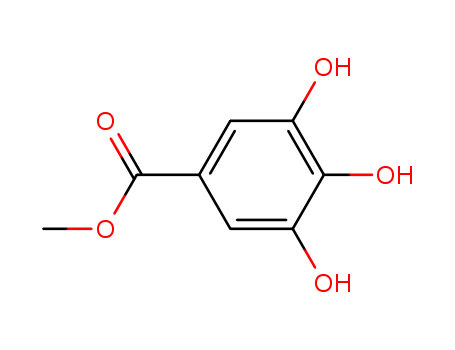
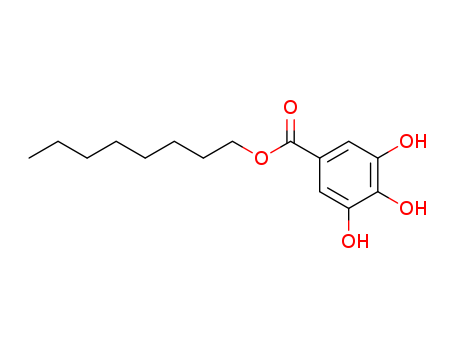
- 3,4,5- three hydroxy benzoic acid
- Gallic acid
- 149-91-7
Quick Details
- ProName: Gallic acid
- CasNo: 149-91-7
- Molecular Formula: C7H6O5
- Appearance: White or white crystalline powder
- Application: Widely used in industry,such as pharma...
- PackAge: 25kg/woven bag
- ProductionCapacity: 800 Metric Ton/Year
- Purity: 99.0%min
- Storage: Avoid moisture and light; hermetically...
- LimitNum: 0 Metric Ton
- Appearance: White or white crystalline powder
- Assay: ≥99.0%
- Loss on drying: ≤10%
- Ignition residue: ≤0.1%
- APHA: ≤180
- Water solubility: Clear
- Turbidity(PPM): ≤10
- Tannic Acid(PPM): ≤1
- Sulphate(PPM): ≤10
- Heavy metal(PPM): /
Superiority
Gallic acid (also known as 3,4,5-trihydroxybenzoic acid) is a trihydroxybenzoic acid, a type of phenolic acid, found in gallnuts, sumac, witch hazel, tealeaves, oak bark, and other plants.The chemical formula of gallic acid is C6H2(OH)3COOH. It is found both free and as part of hydrolyzable tannins. The gallic acid groups are usually bonded to form dimers such as ellagic acid. Hydrolyzable tannins break down on hydrolysis to give gallic acid and glucose or ellagic acid and glucose, known as gallotannins and ellagitannins, respectively.
Gallic acid forms intermolecular esters (depsides) such as digallic and trigallic acids, and cyclic ether-esters (depsidones).
Gallic acid is commonly used in the pharmaceutical industry as a standard for determining the phenol content of various analytes by the Folin-Ciocalteau assay; results are reported in gallic acid equivalents. Gallic acid can also be used as a starting material in the synthesis of the psychedelic alkaloid mescaline.
The name is derived from oak galls, which were historically used to prepare tannic acid. Despite the name, gallic acid does not contain gallium. Saltsand esters of gallic acid are termed "gallates".
Details
Gallic acid is an important component of iron gall ink, the standard European writing and drawing ink from the 12th to 19th centuries, with a history extending to the Roman empire and the Dead Sea Scrolls. Pliny the Elder (23-79 AD) describes the use of gallic acid as a means of detecting an adulteration of verdigris and writes that it was used to produce dyes. Galls (also known as oak apples) from oak trees were crushed and mixed with water, producing tannic acid. It could then be mixed with green vitriol (ferrous sulfate) — obtained by allowing sulfate-saturated water from a spring or mine drainage to evaporate — and gum arabic from acacia trees; this combination of ingredients produced the ink.
Gallic acid was one of the substances used by Angelo Mai (1782–1854), among other early investigators of palimpsests, to clear the top layer of text off and reveal hidden manuscripts underneath. Mai was the first to employ it, but did so "with a heavy hand", often rendering manuscripts too damaged for subsequent study by other researchers.
Gallic acid was first studied by the Swedish chemist Carl Wilhelm Scheele in 1786. In 1818, French chemist and pharmacist Henri Braconnot (1780–1855) devised a simpler method of purifying gallic acid from galls; gallic acid was also studied by the French chemist Théophile-Jules Pelouze(1807–1867), among others.
Gallic acid is a component of some pyrotechnic whistle mixtures.


 Premiumsupplier
Premiumsupplier